
How do you create digital therapies and prescribable apps to improve health outcomes?

With most patients having limited quality time with their doctors, how can digital solutions empower patients to manage their own care, and serve as a “proxy” for the doctor in between visits? At IA Collaborative, we’ve been working with the world’s largest pharmaceutical companies to conceive, design and launch new-to-the-world digital therapies and prescribable apps. The solutions are intuitive, seamless, and connected across the entire healthcare ecosystem from patients to payers, to providers and caregivers. While the digital therapy space is a clear growth market within healthcare, creating and launching solutions requires new collaboration models that balance human-centered design and product strategy, agile development, and a careful navigation of regulatory requirements.
1 / Patient Outcomes: Strengthening the Impact a Drug Can Have
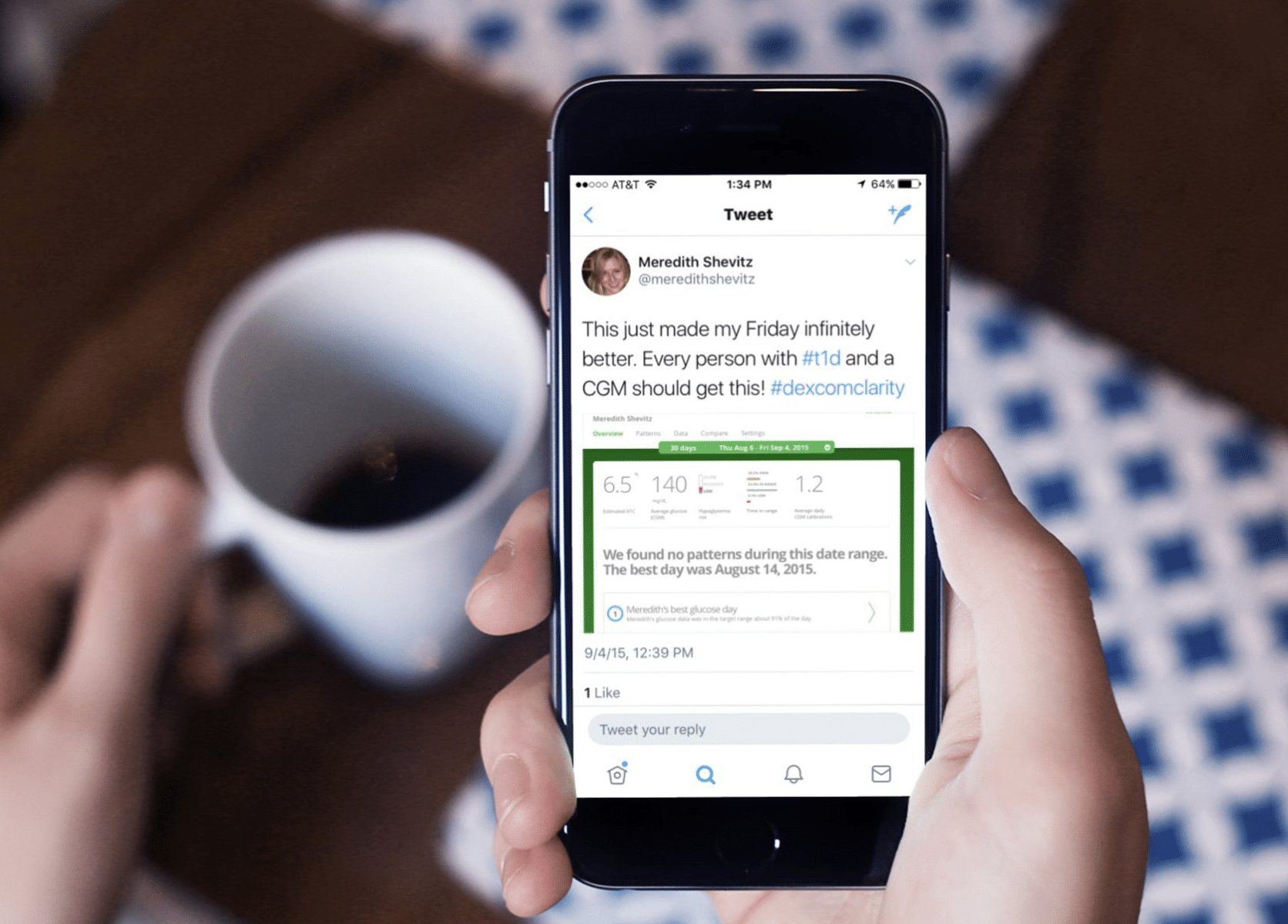
Digtal therapies that capture patient data and provide hyper-personalized support enable pharmaceutical companies to connect directly with patients and improve their outcomes. Digital therapies enable Pharma organizations to differentate themselves while driving consumer preference and loyalty. IA Collaborative works with these organizations to define their ownable opportunity wtihin this $1 Trillion potential market.
2 / Uncovering User Needs & Market Opportunity
From diabetes to dialysis, we’re uncovering unique opportunities for digital solutions to play a role in improving patient care and achieving better health outcomes. Digital solutions provide benefits that doctor’s visits and drugs alone cannot, including support and asynchronous communication between check-ups; objective data vs. self-reported adherence and behavioral data; and enhanced “real-time” collaboration opportunties between doctors, patients, and caregivers.

3 / From Insight to Implementation
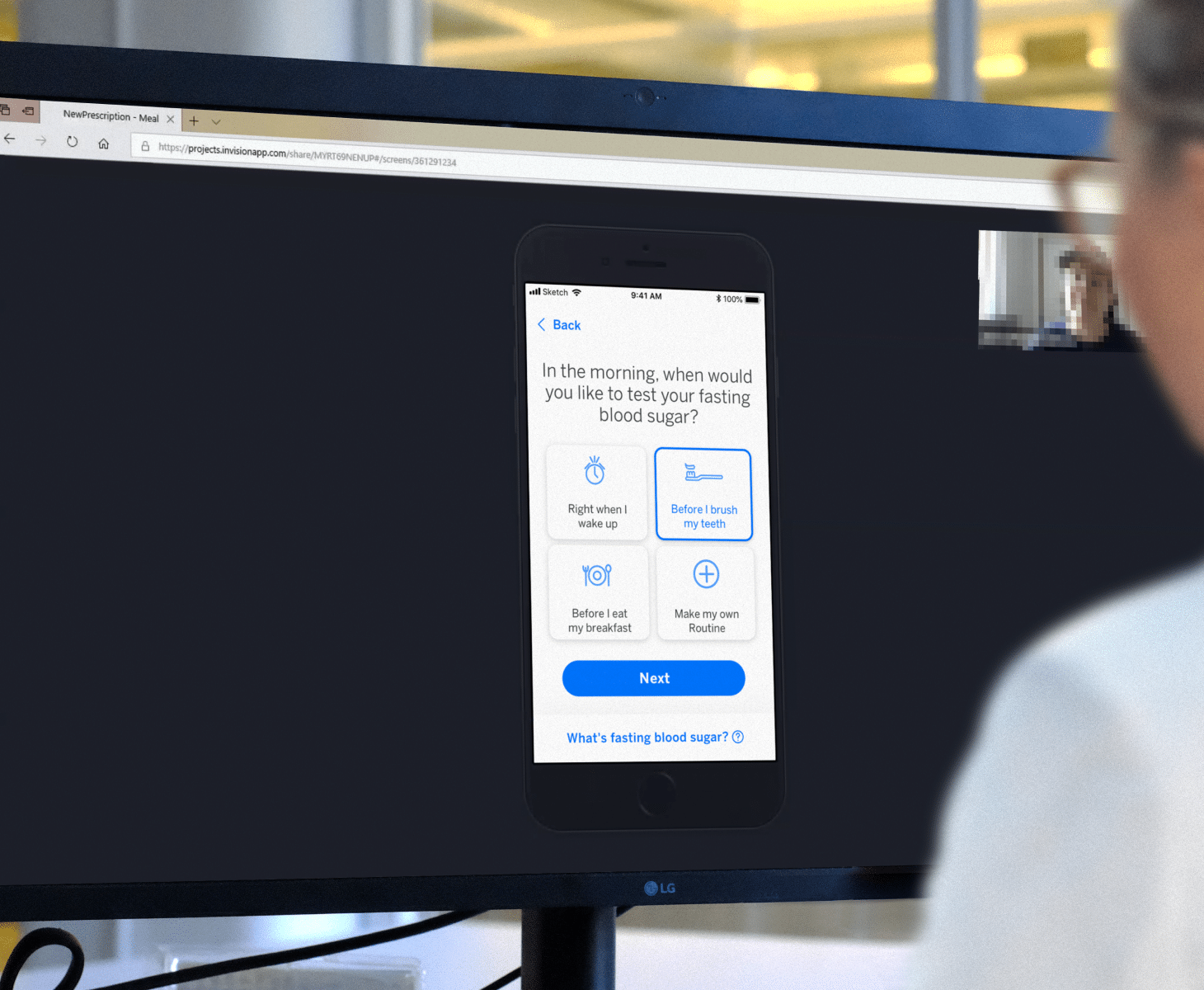
Launching connected digital solutions within large Pharma organizations requires the right organizational infrastructure, a tailored process and collaboration model, and a strategy to drive internal alignment quickly, and iteratively. With long regulatory time horizons, a central challenge is ensuring products are relevant, timely and competitive by the time they hit the market. IA Collaborative helps organizations conduct the right sequence of behavioral design research and usability testing to ensure that products are effective in changing behavior – before they head into clinical trials.

4 / De-Risking Investment
IA Collaborative teams function as an extension of our client’s team, leading product strategy, research and design to move from early prototypes to MVPs to impelmentation. Through this process, we gather data that proves how our digital therapies positively impact adherence, justifying the investment as a mutually beneficial one for our Pharma client partners, providers, payers, and patients. We help “de-risk” the clinical trial process by ensuring concepts have been explored, iterated, co-created with end users and validated through rapid user testing before entering the regulatory process. By defining the precise user experience that will generate the biggest impact, we help you determine whether you should build from within, buy, or partner to create your digital therapy product.
Spark a conversation.
Let’s talk